Humidity Sensor of Graphite
Team Member
Xu Ruizhe, Ma Shunyu, Li Zerui, Wei Heyi
Introduction
Humidity sensors play an essential role in fields such as environmental monitoring, industrial process control, agriculture, and healthcare, where accurate humidity measurements are critical. High-quality sensors are necessary to precisely track humidity levels and variations. Traditionally, materials like metal oxides and polymers have been used, but they often suffer from limitations including slow response times, low sensitivity, and limited accuracy.
In recent years, carbon-based materials have gained significant interest due to their unique physical and chemical properties. They offer remarkable features such as high surface area, excellent electrical conductivity, and strong chemical stability across various environments. These advantages make carbon materials highly promising for developing sensitive and reliable humidity sensors. For instance, carbon nanotubes (CNTs) enhance sensor response speed thanks to their large surface area and porosity, while graphene provides ultra-fast signal transduction owing to its thin structure and superior conductivity. Other forms like carbon black and carbon fiber show strong resistance to humidity-induced degradation, ensuring long-term operational stability.
The humidity sensing capability of carbon-based materials largely stems from their interaction with water molecules. Changes in humidity cause adsorption or desorption of water on the material surface, altering properties such as resistance or capacitance. Sensor performance can further be tailored by adjusting material morphology or engineering defects.
In conclusion, carbon-based materials offer exciting prospects for future humidity sensing technologies. By continuously improving their physicochemical properties through nanotechnology and materials engineering, it is possible to develop sensors with greater sensitivity, faster response, and broader adaptability to complex humidity environments.
Experimental Principle
In this experiment, a layer of graphite was coated onto paper-based substrates. The fiber paper is primarily composed of cellulose, consisting of long chains of glucose units containing hydroxyl (-OH) groups capable of attracting water molecules. When humidity increases, the hydroxyl groups form hydrogen bonds with water molecules, allowing cellulose to absorb moisture. This adsorption process causes the cellulose structure to expand, affecting the spacing and contact points between graphite particles on the surface.
The graphite layer on the paper was applied using a pencil and plays a crucial role in the humidity response of the sensor based on resistance changes. Graphite, composed of layers of carbon atoms and localized electrons, exhibits excellent electrical conductivity. In the resistive humidity sensor, the adsorption of water molecules modifies the graphite network because the adsorbed water molecules alter the connectivity between graphite particles.
Under low humidity conditions, electrical conduction mainly occurs through direct contact between graphite particles, and the sensor exhibits an intrinsic resistance . As humidity rises, a thin layer of water molecules adsorbs onto the surface, initially forming a monolayer. During this monolayer adsorption, water molecules ionize according to:
The generation of and ions facilitates charge transport, enhancing the conductivity and thus decreasing the resistance according to:
where is the effective length and is the cross-sectional area through which conduction occurs.
As humidity further increases, multilayer adsorption takes place, increasing the thickness of the adsorbed water film. This thick water layer impacts the conductivity in two competing ways: on one hand, the increased number of ions continues to promote conducticonduction and decrease resistance; on the other hand, when the water layer becomes thick enough, it can act as an insulating barrier, interrupting direct electrical pathways between graphite particles and causing the resistance to rise. Thus, due to this competition, the sensor’s resistance exhibits a non-monotonic trend with increasing humidity—initially decreasing and subsequently increasing as the humidity surpasses a certain point.
Additionally, the porous structure of the paper significantly influences the sensor's response. Tiny voids within the cellulose fibers can trap water molecules, leading to localized moisture accumulation. This trapped moisture can either enhance ionic conductivity or disrupt the graphite network, depending on the amount and distribution of absorbed water. Consequently, microscopic changes in water content cause corresponding variations in the effective resistance of the sensor. However, because the evaporation of water trapped within micropores is relatively slow, the recovery time of the sensor may be prolonged after humidity levels drop.
Objective
(1) Build a a sealed drying chamber: use a dry box; control the humidity by desiccants and hot water.
(2) Monitor the humidity with sensor: use DHT11 sensor to monitor the humidity and temperature inside the chamber; verify the range of humidity.
(3) Measure the resistance of Graphite sensor: use Arduino to measure the resistance; record the data with Python code.
(4) Data analysis: use Python code to do fitting; get the experimental relationship between Graphite's resistance and humidity; analyze the error.
Equipment Required
DHT11
The DHT11 is a temperature and humidity composite sensor with calibrated digital signal output, suitable for measuring ambient temperature and humidity. It can be coded with Arduino. Here we use this sensor to get the standard humidity.
Basic Specifications
| Parameter | Value |
|---|---|
| Temperature Range | 0 – 50°C |
| Temperature Accuracy | ±2°C |
| Humidity Range | 20 – 90% RH |
| Humidity Accuracy | ±5% RH |
| Sampling Period | ≥ 1 second |
| Signal Output | Digital (single-wire) |
Desiccant
Equipment Required
Alcohol molecules exhibit distinct absorption characteristics in the infrared spectrum due to the vibrational modes of specific functional groups, particularly the hydroxyl (-OH) and methyl (-CH₃) groups. These groups interact with infrared light at specific wavelengths, leading to unique absorption peaks that can be utilized for concentration measurement.
In the near-infrared (NIR) region, the wavelength range between 1250 nm and 1350 nm is particularly sensitive to ethanol concentration. Notably, around 1300 nm, ethanol demonstrates significant variations in absorption intensity that correlate directly with its concentration. This is primarily due to the overtone and combination vibrations of the C-H and O-H bonds, which result in measurable absorption differences as ethanol content changes.
By analyzing these absorption variations, it is possible to accurately determine alcohol concentration in a given sample. The wavelength of 1300 nm is chosen as the optimal measurement wavelength because it provides a clear and distinguishable response to ethanol concentration changes.
Objective
This experiment proposes a non-contact alcohol concentration measurement method by using infrared spectroscopy. This method measures changes in infrared light intensity transmitted through alcohol solutions, using a spectrometer and principal component analysis to achieve high precision measurements.
Advantages of Infrared-Based Alcohol Detection
- Non-contact measurement, reducing contamination.
- High accuracy, based on alcohol-specific infrared absorption characteristics.
- Low cost, with simple photodetection devices.
Equipment Required
- Different concentration of ethanol
- Cuvette
- LAMBDA 850 Spectrometer
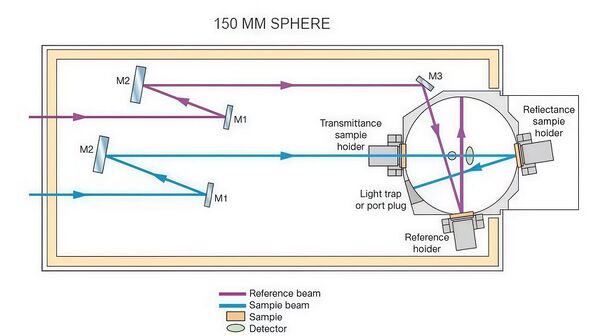
Experiment Set Up
The LAMBDA 850+ UV-Visible-NIR spectrophotometer used for measurements is capable of precise spectral measurements within the wavelength range of 800 nm to 1400 nm, making it suitable for analyzing the absorption characteristics of substances such as alcohol molecules. The device is equipped with both a deuterium lamp and a tungsten-halogen lamp, providing UV and visible/near-infrared light sources, respectively. The high-quality master-engraved holographic grating monochromator ensures precise wavelength control, guaranteeing high-resolution spectral measurements.
The core of the device includes a high-sensitivity R6872 photomultiplier tube (PMT) detector, ensuring accurate signal collection across a broad wavelength range from UV to near-infrared. In the analysis of the effect of alcohol concentration changes on absorption intensity, the LAMBDA 850+ provides high sensitivity and precise measurements.
With this high sensitivity and precision configuration, it provides strong support for the optical property analysis of solutions such as alcohol, especially excelling in peak absorption location and concentration variation analysis.
Experiment Procedures
- Preparation of alcohol solutions at concentrations of 25%, 50% and 75%.


- Measurement of absorption using spectrometer.

- Use Origin software for spectral normalization and baseline correction, and process the alcohol spectra at three different concentrations.(Due to the subtle differences in the spectral data, it is difficult for non-experts to visually distinguish between the spectra of the three alcohol concentrations. Therefore, machine learning-based Principal Component Analysis (PCA) is applied for spectral classification, enabling accurate measurement of alcohol concentration.)